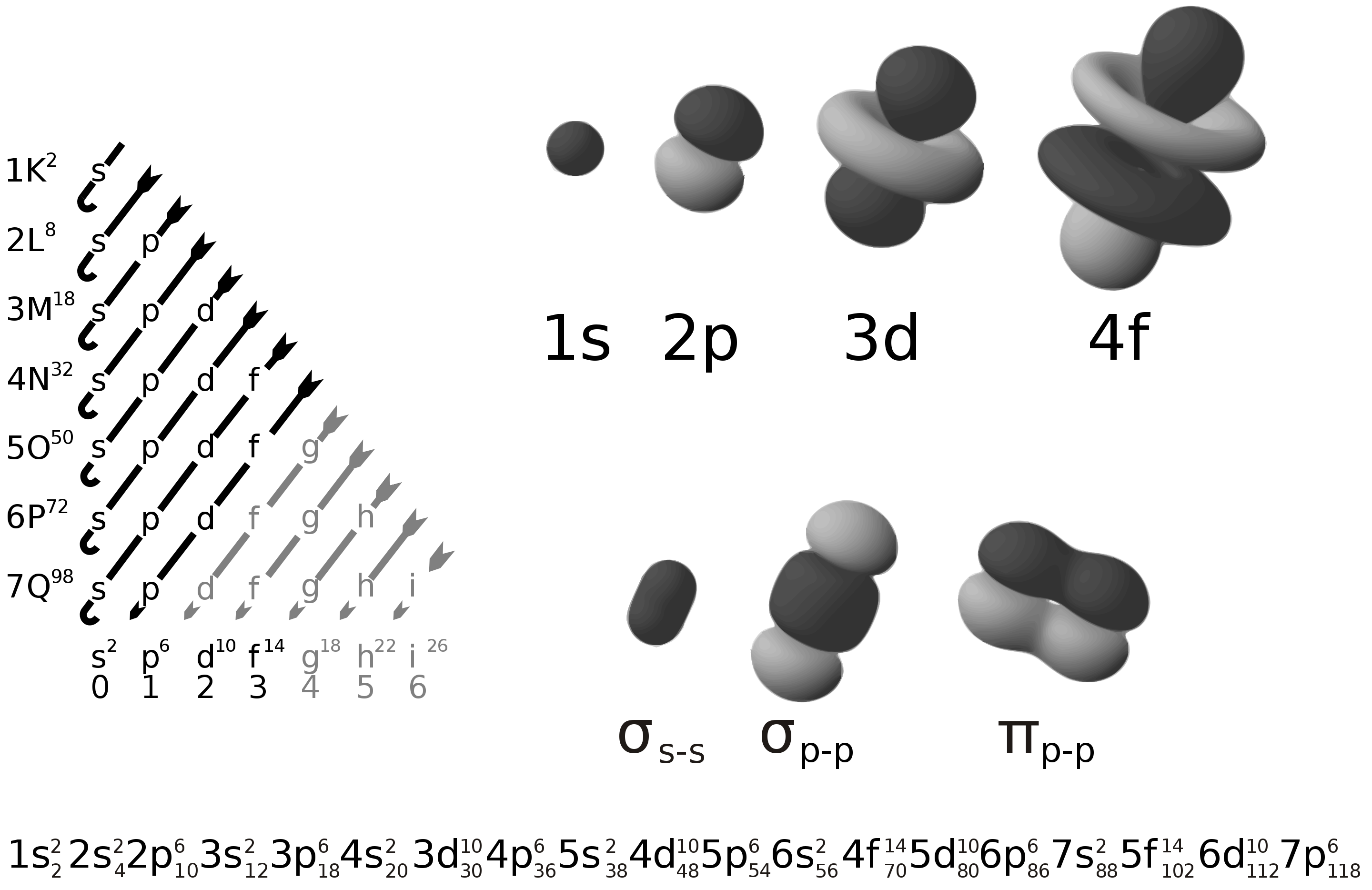
1s2 2s2 2p6 3s2 3p6 Elektronlar Atomun Etrafında Nerede Bulunuyor
The total number of electrons present in the given electronic configuration is 1s22s22p6 = 2+2+6= 10. As, number of electrons = numbers of protons = 10. Hence, the given electronic configuration is of Neon. Suggest Corrections. 4.

1s2 2s2 2p6 3s2 3p6 4s1 3d5 JosephhasKnapp
Which element has the electron configuration of 1s2 2s2 2p6 ? Wayne Breslyn 728K subscribers Join Subscribe Subscribed 383 54K views 3 years ago To figure this out the element with the electron.
Wiswesser S Wikipedia 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
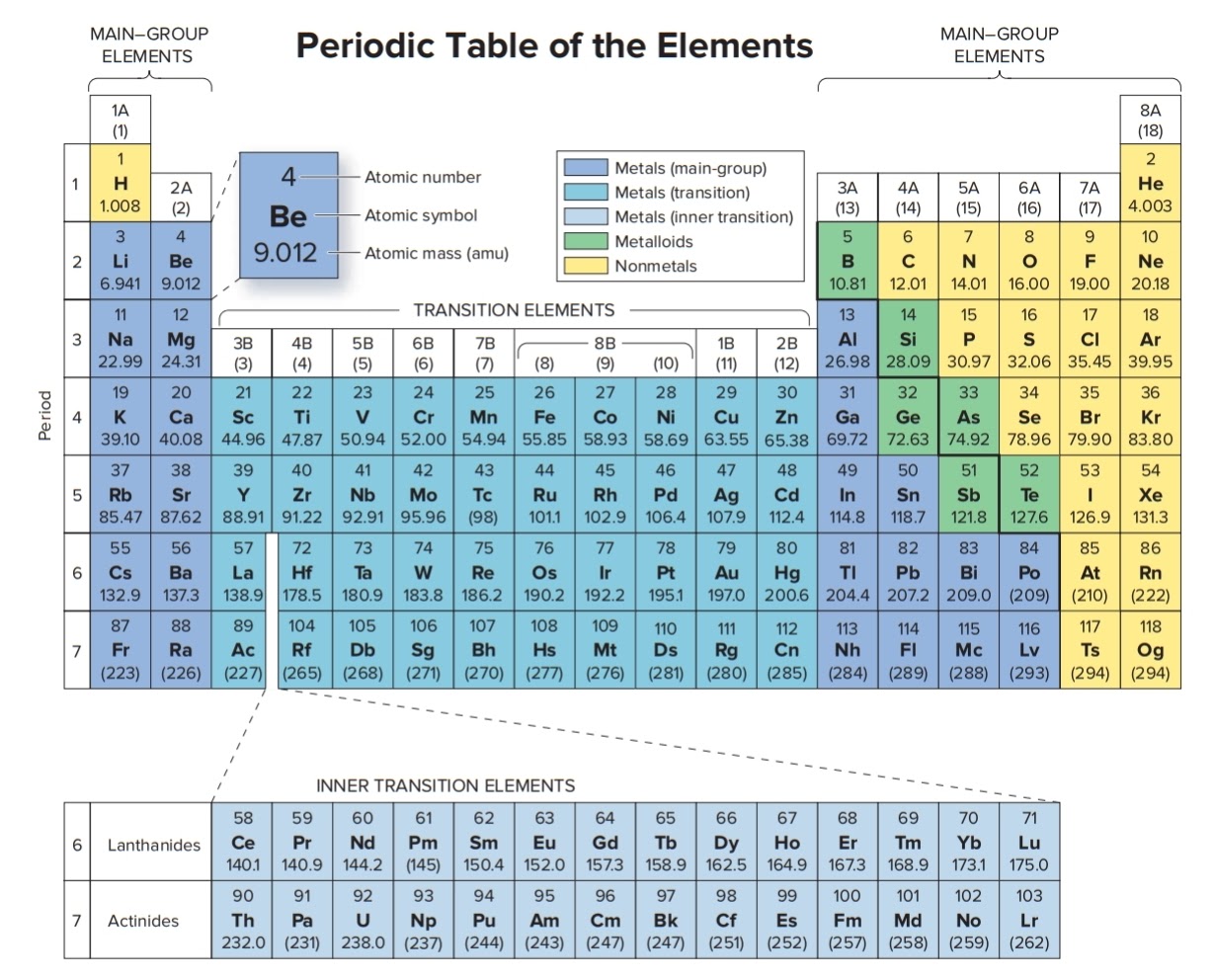
As we will see below, the periodic table organizes elements in a way that reflects their number and pattern of electrons, which makes it useful for predicting the reactivity of an element: how likely it is to form bonds, and with which other elements. Electron shells and the Bohr model
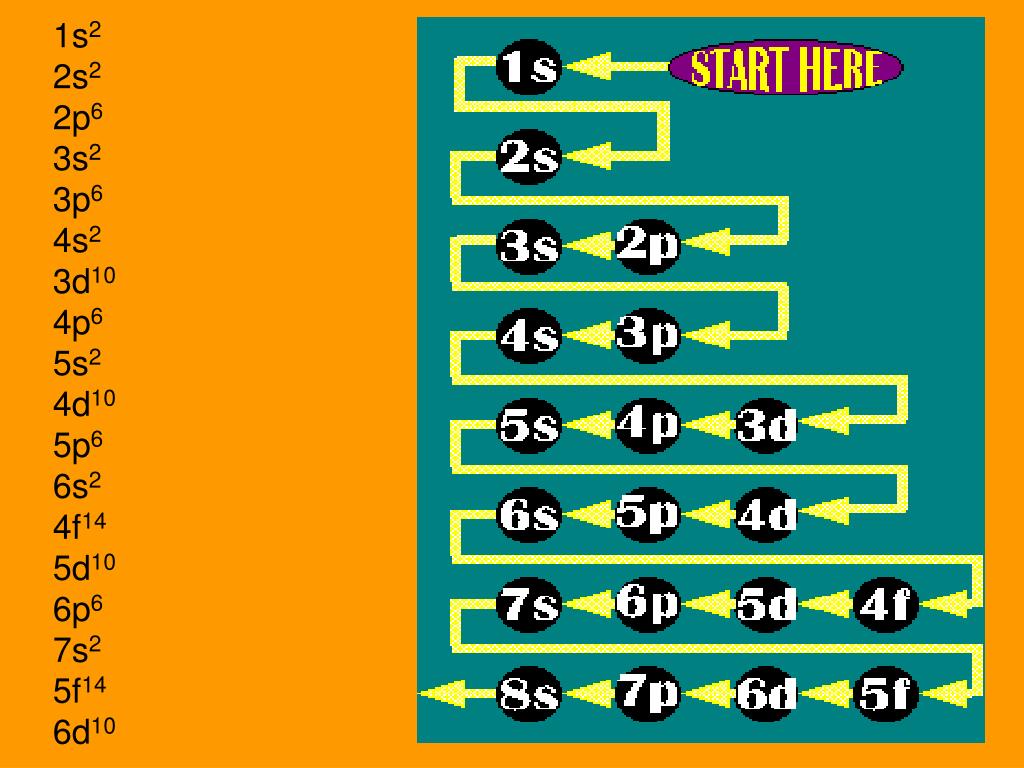
PPT Electron Configuration Notes PowerPoint Presentation, free
atomic physics (or other physical structure) in [1] For example, the electron configuration of the , meaning that the 1s, 2s and 2p subshells are occupied by 2, 2 and 6 electrons respectively. Electronic configurations describe each electron as moving independently in an orbital, in an average field created by all other orbitals.

1s2 2s2 2p6 3s2 3p6 4s1 3d5 KoltenkruwHernandez
1s2 2s2 2p6 3s2 3p6 3d10 4s1. For the Cu+ ion we remove one electron from 4s1 leaving us with: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10. For the Cu2+ ion we remove a total of two electrons (one from the 4s1 and one form the 3d10) leaving us with. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9

Which element has the electron configuration of 1s2 2s2 2p6 3s2 3p6 3d5
So the full electron configuration is 1S2, 2S2, 2P6, and 3S1. When I want to figure out how many valence electrons sodium has, the number of valence electrons would be equal to the number of electrons in the outermost shell, the outermost energy level. For sodium, sodium has the first energy level, second energy level, and the third energy.
1s2 2s2 2p6 3s2 What is 1s2 2s2 2p6 3s2 ?What element has the
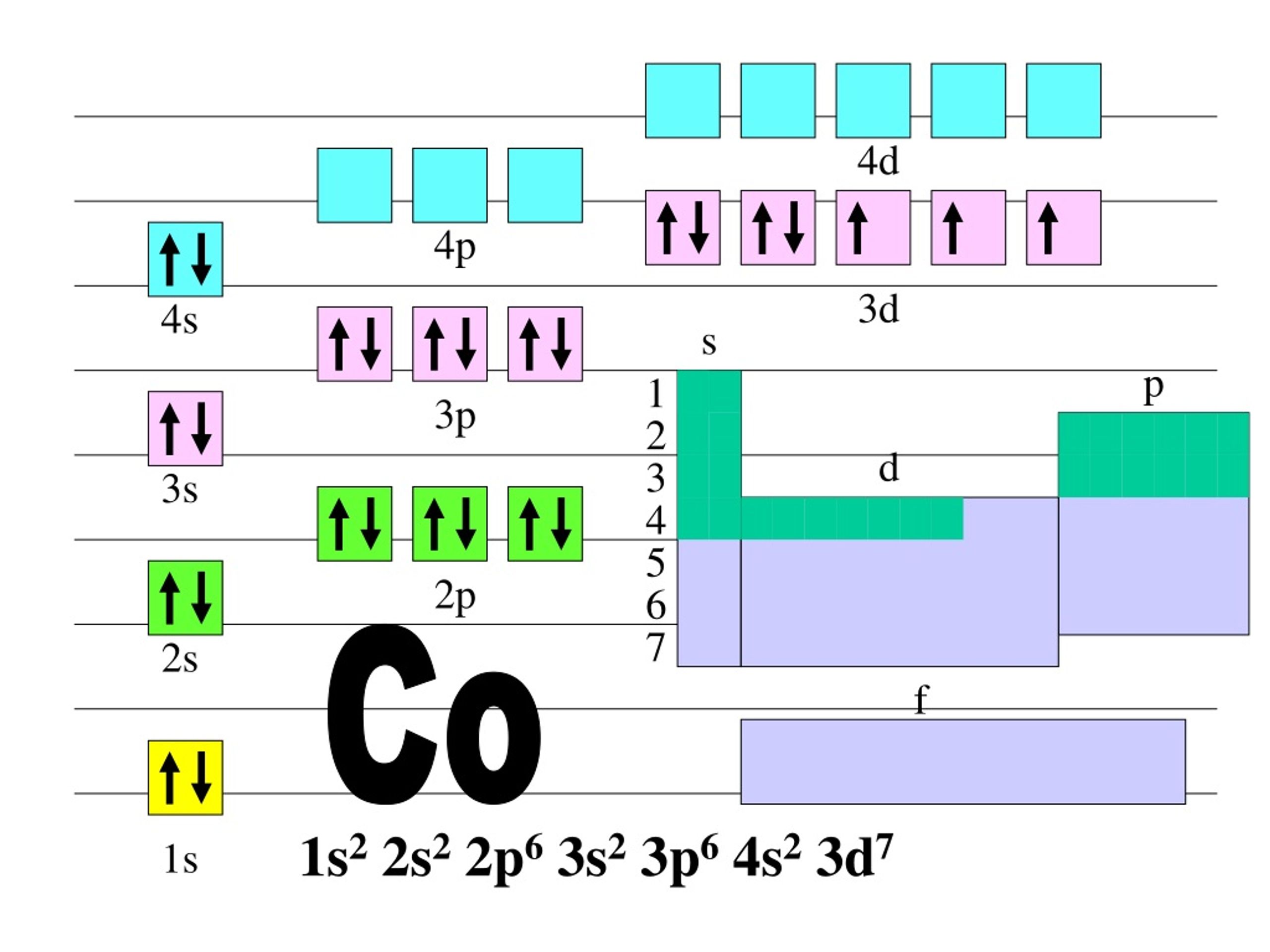
PROBLEM 3.1.12 3.1. 12. In one area of Australia, the cattle did not thrive despite the presence of suitable forage. An investigation showed the cause to be the absence of sufficient cobalt in the soil. Cobalt forms cations in two oxidation states, Co 2+ and Co 3+. Write the electron structure of the two cations. Answer.

Which element has the electron configuration of 1s2 2s2 2p6 3s2 3p6 3d1
Half-filled and fully filled subshell have got extra stability. Therefore, one of the 4s2 electrons jumps to the 3d5 so that it is half-filled (see video below). This give us the (correct) configuration of: 1s2 2s2 2p6 3s2 3p6 3d5 4s1

1S2 2S2 2P1 Boron Neon Lithium Helium Elements, Chemicals and
However, the first part of this long expression, 1s2 2s2 2s6, is the electron configuration of neon. Therefore, to shorten the configuration for silicon she wrote [Ne] 3s2 3p2. All that this means is that silicon has a core structure that is the same as neon, but then has four more electrons (3s2 3p2).

Which element has the electron configuration of 1s2 2s2 2p6 3s2 3p6
So, 1s2 2s2 2p6. I showed you how you could look at the periodic table and kind of run through these electron configurations. For example, this would be 1s1, 1s2 and then 2s2 would be here and then we had six. So 2p6 brings you all the way over to neon. And so for an electron configuration for the elements in the third period, so this would be.

1s2 2s2 2p6 3s2 3p6 4s2 3d10 Element LeonhasHines
1s2 2s2 2p6 3s2 3p6 4s1 is the electronic configuration of the 19th electron of chromium. since the last shell is 4, therefore n=4, l=0(as there is only one orbital in the s sub shell) , m=0 (since we're still in the s sub shell only) and s=+1/2 or s=-1/2(arbitrarily assigned values)
1s2 2s2 2p6 astonishingceiyrs
1s2 2s2 2p6 3s2 3p5: Argon: 1s2 2s2 2p6 3s2 3p6: Potassium: 1s2 2s2 2p6 3s2 3p6 4s1: Calcium: 1s2 2s2 2p6 3s2 3p6 4s2: Mr. Vissers. Send e-mail; This activity was created by a Quia Web subscriber. Learn more about Quia: Create your own activities.

Which element has the electron configuration of 1s2 2s2 2p6 3s2 3p6 3d3
1s2 2s2 2p6 -> neon (Ne). By the way, it's not electricity configuration - it's electronic configuration. The root word is "electron", the orbitals of which is described by 1s2 2s2 2p6. As for the best way to determine the element purely based on its electronic configuration, it is difficult for elements beyond 18 which is argon (Ar). For elements below proton number 18, if the electronic.

Which element has the electron configuration of 1s2 2s2 2p6 3s2 3p3
Question Electronic configuration of first 30 elements with table full explanation Solution H (Hydrogen) 1s1 He (Helium) 1s2 Li (Lithium) 1s2 2s1 Be (Beryllium) 1s2 2s2 B (Boron) 1s2 2s2 2p1 C (Carbon) 1s2 2s2 2p2 N (Nitrogen) 1s2 2s2 2p3 O (Oxygen) 1s2 2s2 2p4 F (Fluorine) 1s2 2s2 2p5 Ne (Neon) 1s2 2s2 2p6 Na (Sodium) 1s2 2s2 2p6 3s1

1s2 2s2 2p6 astonishingceiyrs
This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.

Use the electron configuration mnemonic to complete the table below
Like you can write 1s2 2s1 out as [He] 2s1, which basically means the electron configuration of He (the previous row's noble gas), and then whatever else is outside the square brackets. It doesn't save much space here, but imagine if you were writing the electron configuration for say iron out, it takes much less space to write out [Ar] 4s2 3d6 than to write 1s2 2s2 2p6 3s2 3p6 4s2 3d6